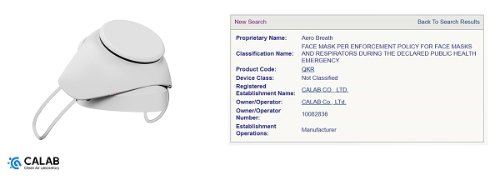
CALAB Co., Ltd. announced on the 20th that the electronic mask "Aero Breath" is about to enter the U.S. with approval from the U.S. Food and Drug Administration (FDA).
According to CALAB, Aero Breath is in the final stage of the certification process until FCC certification along with the approval of the US FDA, and after completion, it will be launched on Amazon, the world's e-commerce platform.
Aero Breath is equipped with a high-performance filter that blocks ultrafine dust and suspended bacteria with an efficiency of up to 99.99%, and a high-performance fan that supplies more than 100L of air that is not insufficient even during exercise. It is an electronic mask that can be used for up to 3 months with one filter.
In particular, high-performance filters were developed through CALAB's own filter design technology, which specializes in air quality improvement, to increase the performance of blocking harmful substances.
According to CALAB, aerobres blocks up to 99.9% of ultrafine dust of 0.3μm level and removes 99.9% of airborne bacteria (rich pneumonia bacteria). In addition, harmful substances such as harmful gases and odors are removed from the respiratory tract.
A CALAB official said, "Aero Breath is conducting various certifications, including FDA approval, for the purpose of overseas expansion as well as KC certification in Korea," adding, "It is an electronic mask that considers economic aspects due to its long filter life."
According to CALAB, Aero Breath is in the final stage of the certification process until FCC certification along with the approval of the US FDA, and after completion, it will be launched on Amazon, the world's e-commerce platform.
Aero Breath is equipped with a high-performance filter that blocks ultrafine dust and suspended bacteria with an efficiency of up to 99.99%, and a high-performance fan that supplies more than 100L of air that is not insufficient even during exercise. It is an electronic mask that can be used for up to 3 months with one filter.
In particular, high-performance filters were developed through CALAB's own filter design technology, which specializes in air quality improvement, to increase the performance of blocking harmful substances.
According to CALAB, aerobres blocks up to 99.9% of ultrafine dust of 0.3μm level and removes 99.9% of airborne bacteria (rich pneumonia bacteria). In addition, harmful substances such as harmful gases and odors are removed from the respiratory tract.
A CALAB official said, "Aero Breath is conducting various certifications, including FDA approval, for the purpose of overseas expansion as well as KC certification in Korea," adding, "It is an electronic mask that considers economic aspects due to its long filter life."
